Scientific Commentary
Innate Defense Role of Extracellular Vesicles: The Critical Role of Phosphatidylserine in Combating Apoptotic Mimicry Viruses 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 2
Received: 26 Mar., 2024 Accepted: 16 Apr., 2024 Published: 27 Apr., 2024
The paper titled "Phosphatidylserine-exposing extracellular vesicles in body fluids are an innate defence against apoptotic mimicry viral pathogens" was published in the journal "Nature Microbiology" on March 25, 2024. Authored by Rüdiger Groß, Hanna Reßin, and Janis A. Müller, the research was conducted at the Institute of Molecular Virology at the University Medical Center Ulm, Germany, and the Institute of Virology at Philipps University of Marburg, Germany. The study reveals that extracellular vesicles (EVs) in body fluids combat viral infections by exposing phosphatidylserine (PS), which disrupts viral attachment and entry, thus serving an antiviral function. The results indicate that these EVs can effectively inhibit several viruses, including Dengue, West Nile, Chikungunya, Ebola, and Vesicular stomatitis viruses. However, they show lower inhibitory effects on SARS-CoV-2, HIV-1, Hepatitis C, and Herpes viruses due to these pathogens utilizing different receptor mechanisms for cell infection.
1 Interpretation of Experimental Data
Researchers collected critical data through lipidomic analysis, flow cytometry, and viral infection experiments. The experimental data indicate that the exposure of phosphatidylserine is crucial to the antiviral properties of extracellular vesicles (EVs). Additionally, the study shows that liposomes containing synthetically produced phosphatidylserine can also inhibit viral infections.
Figure 1 illustrates the role of phosphatidylserine (PS) in Zika virus (ZIKV) and other viral infections. Panel a shows the process where phosphatidylserine decarboxylase (PSD) converts PS in the viral envelope into phosphatidylethanolamine (PE). Panel b indicates that after PSD treatment, the infection rates of ZIKV, HIV-1, HSV-1, and HSV-2 significantly decrease. Panels c and d explore the effects of liposomes with different PS contents on inhibiting ZIKV infection, revealing that the inhibitory effect on infection significantly increases with higher PS content. The data reveal the crucial role of PS in the viral infection process, establishing it as a potential antiviral target.
 Figure 1 NPS exposure in the viral envelope of ZIKV is essential for infection |
Figure 2 investigates the effects of liposomes containing phosphatidylserine (PS) in inhibiting Zika virus (ZIKV) infection. Panel a shows that adding PS liposomes either prior to or simultaneously with the virus significantly inhibits ZIKV infection. Panel b demonstrates that PS liposomes exhibit a concentration-dependent inhibitory effect across different viral inoculation doses (MOI). Panel c indicates that individual headgroup small molecules do not possess significant antiviral activity. Panels d and e reveal that liposomes with a higher PS content have stronger antiviral effects. Panels f and g, using fluorescence quantification, show that PS liposomes can reduce viral attachment and increase the attachment of the liposomes themselves. Panel h, through fluorescence microscopy images, visually displays the competitive inhibitory effect of the liposomes on the virus.
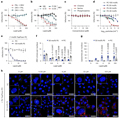 Figure 2 PS-containing vesicles interfere with virion attachment |
Figure 3 explores the levels and characteristics of phosphatidylserine (PS) in extracellular vesicles (EVs) across different body fluids. Panel a describes the workflow for purifying EVs using Tangential Flow Filtration (TFF) and BE-SEC. Panel b confirms the enrichment of characteristic EV proteins in different samples through Western Blot analysis. Panel c shows the content of major phospholipids in EVs from various body fluids, finding that semen and urine have the highest proportions of PS. Panels d-f indicate that EVs from semen and urine have significantly higher contents of PS and PE compared to those from blood. Panel g reveals that the PS+PE/PC ratios are higher in semen, saliva, and urine. Panel h uses flow cytometry to detect subpopulations of EVs exposing PS. Panel i displays the proportion of PS and TSPN positive vesicles in semen EVs. The results suggest significant fluid-specific differences in the lipid composition and PS exposure of EVs.
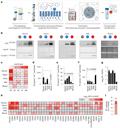 Figure 3 EVs from body fluids expose PS at varying levels |
Figure 4 investigates how extracellular vesicles (EVs) rich in phosphatidylserine (PS) interfere with the viral infection mechanisms. Panel a shows that EVs from semen and other body fluids more effectively inhibit Zika virus (ZIKV) infection compared to control liposomes. Panels b-c, using fluorescence microscopy, confirm that semen EVs effectively reduce viral attachment. Panels d-e reveal that PS-rich EVs block ZIKV infection by binding to the Axl receptor. Panels f-i present "shaving" experiments conducted after treating EVs with phospholipase D, finding that removing PS exposure significantly reduces the antiviral activity of EVs, while replenishing PS restores this capability. This study highlights the critical role of PS in the antiviral mechanisms of EVs.
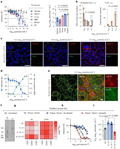 Figure 4 PS-rich EVs from body fluids interfere with viral apoptotic mimicry |
Figure 5 evaluates the inhibitory effects of semen extracellular vesicles (EVs) on Zika virus (ZIKV) infection in reproductive tract cells and tissues. Panel a shows that both semen EVs and seminal plasma (SP) effectively inhibit ZIKV infection in primary human foreskin fibroblasts, while control liposomes do not show significant inhibitory effects. Panel b uses immunofluorescence imaging to display the infection status under different treatment conditions. Panel c further assesses the inhibitory effect of semen EVs and SP on ZIKV in ex vivo vaginal tissue blocks from two donors, finding that semen EVs significantly reduce viral genome levels, confirming their potential application in preventing the spread of ZIKV.
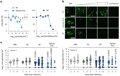 Figure 5 Semen EVs inhibit ZIKV infection of primary cells and tissues from the anogenital tract |
Figure 6 evaluates the inhibitory effects of semen extracellular vesicles (EVs) and liposomes containing phosphatidylserine (PS) or phosphatidylcholine (PC) against various viruses. Panel a shows that semen EVs and PS liposomes significantly inhibit viruses that infect via apoptotic mimicry mechanisms (such as ZIKV, DENV, WNV, CHIKV, EBOV, and VSV), while PC liposomes show no significant inhibitory effect. Panel b demonstrates that for viruses that rely on non-lipid receptors (such as SARS-CoV-2, HIV-1, HSV-1, HSV-2, HCMV, and HCV), the inhibitory effects of semen EVs and PS liposomes are weaker. The results indicate that semen EVs and PS liposomes have specific antiviral activity against viruses that employ apoptotic mimicry.
 Figure 6 EVs specifically inhibit viral pathogens depending on apoptotic mimicry |
2 Insight of Research Findings
Extracellular vesicles (EVs) enriched with phosphatidylserine (PS) significantly reduce viral attachment by competing with viruses for cellular receptors. There are variations in the PS content of EVs from different sources. Data charts illustrate the effectiveness of PS-enriched extracellular vesicles and liposomes in preventing viral infections.
3 Evaluation of the Research
This study validates the theory of extracellular vesicles (EVs) as an innate antiviral mechanism. Experiments have shown that EVs exposing more phosphatidylserine (PS) are most effective at blocking viruses that utilize apoptotic mimicry mechanisms. Additionally, the research also demonstrates the differences in antiviral activity among various types of vesicles and liposomes.
4 Concluding Remarks
The conclusion drawn from the research is that extracellular vesicles (EVs) exposing phosphatidylserine (PS) as a defense mechanism in body fluids may be a potentially effective means of combating specific viruses. Synthetic liposomes that mimic this mechanism have the potential to be used in developing new antiviral therapies.
5 Access the Full Text
Groß, R., Reßin, H., von Maltitz, P. et al. Phosphatidylserine-exposing extracellular vesicles in body fluids are an innate defence against apoptotic mimicry viral pathogens. Nat Microbiol 9, 905–921 (2024). https://doi.org/10.1038/s41564-024-01637-6.
Acknowledgement
Thanks to the open access policy of "Nature Microbiology," readers can freely share this valuable research. Thanks to the excellent research work of the von Maltitz, P. (corresponding author) research team, providing a very good case study for the research community. If the reviewers' understanding and perspectives on the research data, results, and evaluations differ from the authors' intentions, I sincerely apologize.
.png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Manman Li
Related articles
. Innate defense
. Extracellular vesicles
. Phosphatidylserine
. Combating apoptotic
. Mimicry viruses
Tools
. Post a comment